
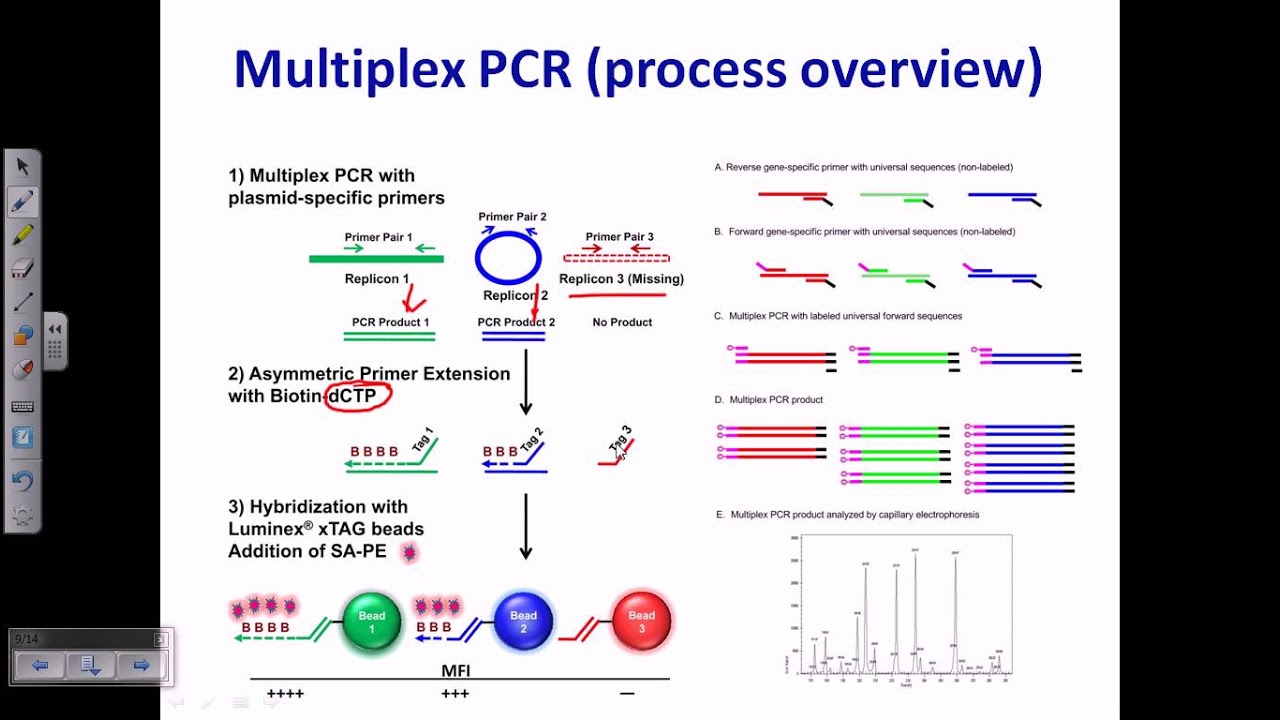
This can be controlled by implementing hot start PCR which allows primer extensions to be blocked until the optimal temperatures are met. Similarly, primer dimers form complexes which decreases the amount of copy number amplifications obtained.
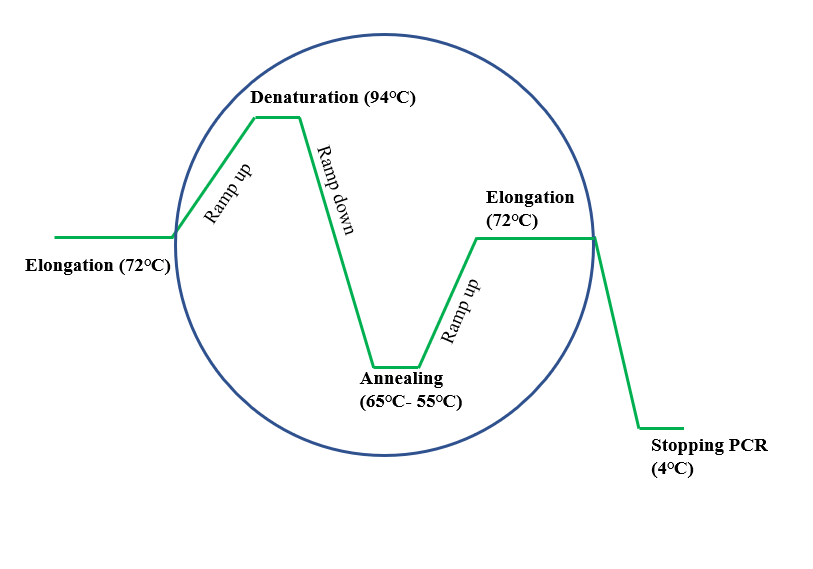

Mis-priming greatly impedes and reduces the efficiency of PCR amplification through actively competing with the target sequences for amplification. These non-specific primer complexes, which are in excess in the mixture, are the cause behind the synthesis of by-products such as primer dimer and mis-priming. In conventional PCR, lower temperatures below the optimal annealing temperature (50-65 ☌) results in off target modifications such as non-specific amplifications where primers will bind non-specifically to the nucleic acid. This reduces annealing time, which in turn reduces the likelihood of non-specific DNA extension and the influence of non-specific primer binding prior to denaturation. In hot start PCR, some of the reagents are kept separate until the mixture is heated to the specific annealing temperature. During the PCR procedure, DNA polymerase will extend any piece of DNA with bound primers, generating target products but also nonspecific products which lower the yield. In conventional PCR, the reaction mix is completed at room temperature, and due to DNA polymerase activity, primers may form primer dimers or anneal to DNA non-specifically. It uses DNA polymerase, which is slightly active at low temperatures. The specific segments of DNA is amplified over three processes, denaturation, annealing and extension – where the DNA strands are separated by raising the temperature to the optimal from room temperature before primers bind and polymerase aligns nucleotides to the template strand. Polymerase chain reaction (PCR) is a molecular biology technique used to amplify specific DNA segments by several orders of magnitude. Procedure of traditional polymerase chain reaction (PCR) This is of utmost importance in diagnostic applications of PCR or RT-PCR. Inhibiting formation of non-specific PCR products, especially in early cycles, results in a substantial increase in sensitivity of amplification by PCR. These modifications work overall to ensure that specific enzymes in solution will remain inactive or are inhibited until the optimal annealing temperature is reached. Through these additional methods, hot start PCR is able to decrease the amount of non-specific amplifications which naturally occur during lower temperatures – which remains a problem for conventional PCR. Some ways to complete reaction mixes at high temperatures involve modifications that block DNA polymerase activity in low temperatures, use of modified deoxyribonucleotide triphosphates (dNTPs), and the physical addition of one of the essential reagents after denaturation. Non-specific binding and priming or formation of primer dimers are minimized by completing the reaction mix after denaturation. However, it utilizes additional heating and separation methods, such as inactivating or inhibiting the binding of Taq polymerase and late addition of Taq polymerase, to increase product yield as well as provide a higher specificity and sensitivity. Hot start PCR follows the same principles as the conventional PCR - in that it uses DNA polymerase to synthesise DNA from a single stranded template. Many variations and modifications of the PCR procedure have been developed in order to achieve higher yields hot start PCR is one of them. Hot start PCR is a modified form of conventional polymerase chain reaction (PCR) that reduces the presence of undesired products and primer dimers due to non-specific DNA amplification at room (or colder) temperatures.


 0 kommentar(er)
0 kommentar(er)
